X-ray absorption spectroscopy (XAS) starts with the excitation of core shell electrons of a specific element in a given material. Since each element on the periodic table has a unique number of protons in the nucleus, the binding force is unique. Hence, one specific element can be probed by tuning the X-ray energy to the binding energy of the electron from the element of interest. This technique is very useful in determining local atomic structures and oxidation states.
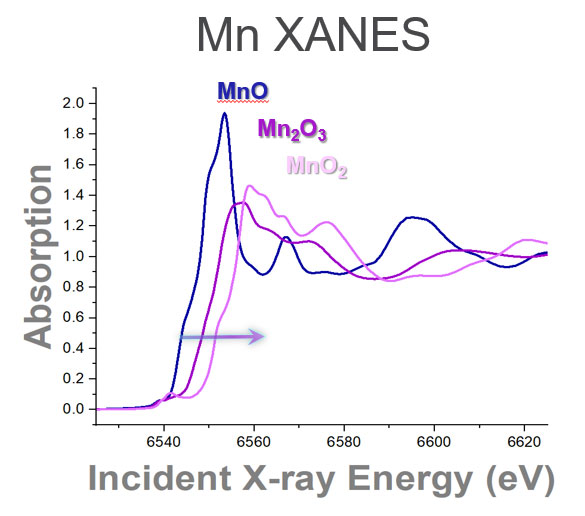
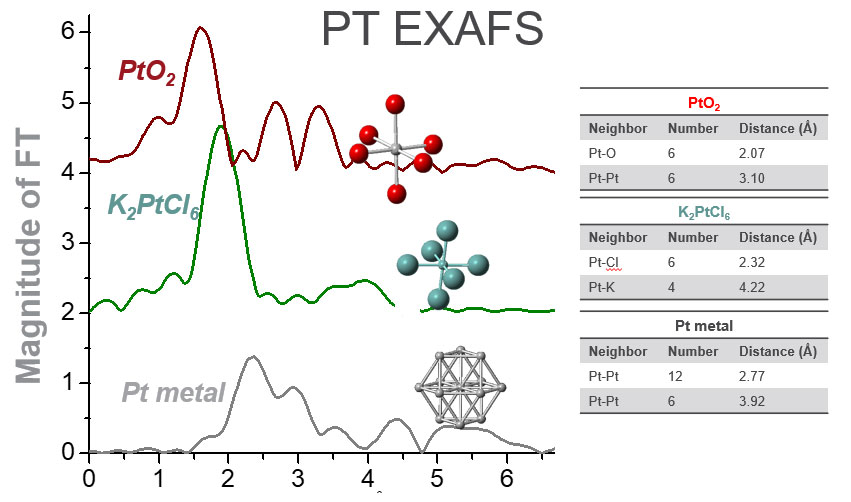
As X-ray energy of the incident beam is scanned through the absorption edge of the material, additional information is obtained about the average local atomic structure of the element of interest. By taking the Fourier Transform of this signal, a unique spectrum is revealed that is related to the number, type, and distance to the neighboring atoms. An example for Pt is shown below. As shown below for Mn, the details of the binding energy vary depending on the element's oxidation state.
The Spectroscopy Group beamlines can reach most elements on the periodic table from P and above with sample concentrations as low as a few hundred ppm.
Sample requirements are minimal. We can work with solids, liquids, and gasses.
|



